Nowadays, many people like to keep pets dogs. dogs live indoors for a long time, lack of sunlight and exercise, and their immunity is weakened, and they are very susceptible to diseases. of. Canine distemper is a highly transmitted disease among dogs. Once infected with canine distemper, the mortality rate is more than 80%.
Infection of the central nervous system (CNS) is the most serious complication of canine distemper, leading to various neurological diseases, often with poor prognosis. Often, neurological symptoms occur in the absence of systemic signs and are associated with demyelinating lesions. However, not all canine distemper cases have neurological symptoms, and there are certain strain differences. For example, the R252 and A75-17 strains currently found mainly cause demyelinating leukoencephalitis, and Snyder Hill mainly causes acute poliomyelitis.
On the one hand, humoral immunity and cellular immunity are crucial in defending against canine distemper virus infection, and the lack of effective humoral immunity at the initial stage may be fatal. On the other hand, it is possible that the presence of antibodies and deposition of immune complexes during the systemic immune response may facilitate viral spread in CNS endothelial cells. In this article, we describe in detail how the virus infects the nervous system and the mechanism of demyelination.
After viral infection, the It appears in the phagocytic cells, and then migrates to lymphoid and hematopoietic tissues (eg: spleen, thymus, lymph nodes, bone marrow), resulting in lymphopenia and immunosuppression, which in turn provides the basis for secondary bacterial infection. Absence or insufficiency of humoral immunity during viral infection promotes secondary viremia, which develops leading to infection of epithelial cells, mesenchymal cells, and the central nervous system (CNS). In the chronic phase, the immune system gradually recovers.
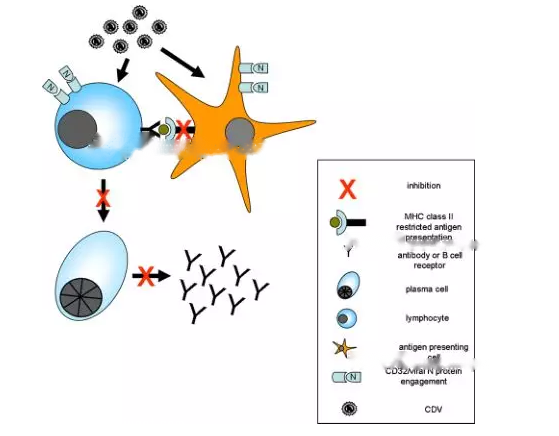
Figure 1. Mechanisms of CDV infection-induced immunosuppression: viral infection and viral N protein/CD32 binding lead to reduced antigen presentation and Interferes with the maturation of dendritic cells and B cells, resulting in a marked reduction in plasma cell formation and immunoglobulin production.
Blood route: virus infects monocytes → Pass through the blood-brain barrier → Infect epithelial and endothelial cells native to the brain → Spread in the brain with cerebrospinal fluid (CSF) → Infect glial cells and neurons.
Other pathways: CDV infects neurons located in the olfactory mucosa → then the virus infects the olfactory glomerulus along the olfactory nerve fibers → transmits to the deeper CNS organs.
The virus recognizes and infects cells through the cellular receptors CD150 and PVRL4, but the expression of these cellular receptors in the CNS is very limited. In infected oligodendrocytes, only CDV RNA was detected but no CDV protein was detected, indicating that CDV was only transcribed but not translated in infected oligodendrocytes. The characteristics of limited infection and viral genes are only transcribed but not translated, which also confirms the fact that no detoxification occurs in the late stage.
Induction of microglia after CDV infection of the CNS Activation→secreting toxin factors (TNF-α, oxygen free radicals)→inhibiting the growth of oligodendrocytes and myelin, causing damage to oligodendrocytes and demyelination.
The myelin sheath is wrapped around the nerve cell axon a layer of film. There are three types of glial cells in the central nervous system (CNS): oligodendrocytes, astrocytes, and microglia, all of which are involved in the formation of myelin. Among them, abnormal oligodendrocytes lead to demyelinating lesions of the central nervous system, neuronal damage or mental diseases; microglia are important immune cells in the CNS; astrocytes provide nutrition for nerve cells.
Another bystander mechanism of macrophage interaction with antiviral antibodies is an important cause of chronic inflammatory demyelination. During chronic viral infection, the immune response gradually recovered, CD4+ cells infiltrated around the blood vessels, followed by the recruitment of a large number of plasma cells, and the synthesis of intrathecal antibodies was vigorous. CDV antibodies in blood and cerebrospinal fluid bind to infected cells and bind to Fc receptors on adjacent macrophages, inducing the release of a large number of oxygen free radicals, causing the destruction of oligodendrocytes or myelin sheaths, resulting in demyelination disease, causing neuronal damage or psychiatric diseases.
Virus-restricted infection: in oligosynaptic In cells, viral genes replicate continuously, but do not express proteins, which can escape recognition by the immune system and can also trigger new demyelinating lesions.
Non-cytolytic selective spread: CDV infection spreads in a non-cytolytic form, releasing a very limited amount of virus. In this way, CDV escapes immune surveillance.
In simple terms, early demyelination is mediated by viruses, and late stage demyelination is mediated by bystander mechanisms involving antigens. Enhanced, antiviral antibodies that bind infected cells bind to phagocytes to further damage myelin and trigger neurological symptoms. Restrictive infection with the virus results in greatly reduced viral protein expression and fewer detectable antigens. Non-cytolytic transmission limits viral release, allowing the helper virus to escape the host's immune mechanisms and persist.
According to this characteristic of canine distemper infection, the prevention and protection of canine distemper can provide a good immune defense for dogs in the early stage of canine distemper infection, which is very important. However, when the dog is in the late stage of canine distemper, the presence of antibodies increases the damage to the nervous system, so it is not recommended to inject monoclonal antibody treatment in the late stage of canine distemper. Humoral immunity runs through the whole process of canine distemper infection, and the development of the disease is tracked through antibody detection, which helps to formulate and change treatment plans in a timely manner.
References
1. Carvalho OV, Botelho CV, Ferreira CG, et al. Immunopathogenic and neurological mechanisms of canine distemper virus. Adv Virol. 2012;2012:163860.
2. Vandevelde M, Zurbriggen A. Demyelination in canine distemper virus infection: a review. Acta Neuropathologica. 2005;109(1):56–68.
3. Botteron C , , Zurbriggen A , , Griot C , , et al. Canine distemper virus-immune complexes induce bystander degeneration of oligodendrocytes.[J]. Acta Neuropathologica, 1992, 83(4):402.
4.Rima BK , Duffy N , Mitchell WJ , et al. Correlation between humoral immune responses and presence of virus in the CNS in dogs experimentally infected with canine distemper virus[J]. Archives of Virology, 1991, 121(1-4):1.
5.Beineke A , Puff C , Seehusen F , et al. Pathogenesis and immunopathology of systemic and nervous canine distemper[J]. Veterinary Immunology & Immunopathology, 2009, 127(1-2):0-18 .
6. Lempp C, Spitzbarth I, Puff C, et al. New aspects of the pathogenesis of canine distemper leukoencephalitis. Viruses. 2014;6(7):2571-601. Published 2014 Jul 2. doi:10.3390/v6072571
Here is a reminder that shit officials should pay attention to dogs For good health, prevention is better than cure. Don't wait until the dog is sick to pay attention to it. When the disease reaches an advanced stage, treatment may not necessarily save the pet's life.
![[Dog Training 5] The training method of pet dog dining etiquette](/static/img/12192/12192_1.jpg)




